Abstract
As a common environmental endocrine disruptor, monobutyl phthalate (MBP) has been connected to reports of ROS accumulation, sperm destruction and reproductive damage. However, the specific mechanism of reproductive injury caused by MBP remains uncertain. Ferroptosis is a non-apoptotic, controlled oxidative damage-related cell death that is usually connected with reactive oxygen species and lipid peroxidation. In this work, to evaluate the mechanism of MBP-induced ferroptosis in reproductive damage, bioinformation analysis and experimental validation were used. Based on bioinformatics analysis, the interleukin-6 (IL-6) and signal transducer and activator of transcription 3 (STAT3) genes may be involved in the tumor necrosis factor (TNF) signaling pathway, which controls inflammation. Experimental study validated the significance of IL6 and STAT3 in MBP-induced ferroptosis. Western blotting and quantitative real-time PCR revealed that Acyl-CoA Synthetase Long Chain Family Member 4 (ACSL4), Tumor necrosis factor-α (TNF-α), IL6, and STAT3 were all elevated with treatment of MBP, but Glutathione peroxidase 4 was significantly decreased. To determine the participation of IL6/STAT3, we added the ferroptosis inhibitor Ferrastain-1 (Fer-1) and the IL6/STAT3 pathway inhibitor Angoline. In conclusion, we found that MBP induced ferroptosis in TM3 cells to damage male reproductive system through the TNF/IL6/STAT signal pathway, resulting in lipid peroxidation and iron metabolite degradation.
INTRODUCTION
According to the research, between 8 and 12 percent of couples worldwide experience infertility, with male factors accounting for more than 50 percent of the major or contributing causes (Agarwal et al., 2021). Despite the fact that there are several reasons of sterility, including hereditary, acquired, and idiopathic variables, its exact etiology remains unclear. Toxic chemical exposure at work or in the environment has been recognized as a potential risk factor for male infertility, owing to the fast growth of scientific research. Furthermore, studies have found that environmental endocrine disruptors have a significant impact on it (Ma et al., 2019; Amir et al., 2021; Yilmaz et al., 2020).
As a ubiquitous environmental endocrine disruptor, phthalates (PAEs) are often used as plasticizers to improve the plasticity and flexibility of a number of commercial and industrial products (Zhang et al., 2021). The interactions between PAEs and polymer molecules in bone make it easier to release into the environment. Individuals are finally exposed to endocrine disrupting chemicals (EDCs) via personal contact, consumption of contaminated food, and inhalation of polluted air (Li and Spade, 2021). Meanwhile, epidemiological studies revealed that sperm quality decreased linearly with increasing PAEs levels and exposure, indicating that PAEs may be detrimental to male sperm (Wang et al., 2020). PAEs can be classified into three types: low molecular weight phthalates; transitional phthalates: Dibutyl phthalate (DBP), Benzyl butyl phthalate (BBP) and Di-(2-ethylhexyl) phthalate (DEHP); and high molecular weight phthalates. The transitional phthalates are regarded as the predominant components in reproductive effects such as decreased fertility, testis weight and alterations in accessory sex organs (Sedha et al., 2021). MBP (the primary metabolite of DBP) accounted for 25.9% of the 14 phthalate metabolites found in the urine of young Chinese people, placing it in first place (Gao et al., 2016; Jin et al., 2021). Once MBP levels in sperm reach a certain threshold, it may interfere with human sperm activity by inhibiting sperm tyrosine phosphorylation, resulting in harm to the reproductive system (Xie et al., 2019). However, the pathways behind MBP-induced testicular injury are poorly known and need more exploration.
Ferroptosis is a non-apoptotic, oxidatively damaged cell death driven by iron buildup and lipid peroxidation. In terms of shape, biology, and genetics, it is distinct from other kinds of cell death (Dixon et al., 2012). A growing amount of evidence demonstrates that mammalian physiological and pathological processes involved in ferroptosis (Zheng and Conrad, 2020; Qiu et al., 2020). A high iron status or excessive iron intake are potential risk factors for reproductive issues (Ng et al., 2019). Despite the fact that multiple studies have stated that ferroptosis occurs in mice testis cells (Bromfield et al., 2019; Meng et al., 2020; Shaygannia et al., 2021), it is still unclear how ferroptosis affects the testes and causes reproductive issues.
TNF is a pro-inflammatory cytokine that plays important roles in mammalian immunity and cellular homeostasis (Brenner et al., 2015). A review of the effect of ferroptosis on inflammation discovered an unbreakable link between ferroptosis and inflammation. Several ferroptosis inhibitors have been shown to have anti-inflammatory effects in specific disorders (Sun et al., 2020b). A bioinformatic analysis, for example, discovered that the TNF signaling pathway is extremely crucial in ferroptosis caused by cerebral hemorrhage (Liu et al., 2021a). As we all know, interleukin-6 (IL6) is not only a cause of ferroptosis but also a key regulator of inflammation. By activating STAT3, IL6 regulates hepcidin to control ferroptosis (Vogt et al., 2021). Although the TNF/IL6/STAT3 signaling pathway may regulate ferroptosis sensitivity, it is unclear whether MBP-induced ferroptosis is regulated by it and what the precise mechanism may be.
Above all, bioinformatic analysis was employed to study the underlying pathways by which MBP induced mouse testicular injury. The goal of this study is to see whether MBP exposure triggered ferroptosis through the TNF/IL6/STAT3 signaling pathway. Our findings will provide a new insight to fully comprehend the male reproductive toxicity of DBP.
MATERIALS AND METHODS
Bioinformation analysis
Screening of microarray data and ferroptosis-related genes
We searched Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/) and the ferroptosis-related database FerrDb (http://www.zhounan.org/ferrdb/index.html) for microarray data and ferroptosis-related genes. First, we obtained the GSE13550, which was based on the GPL1355 platform and contained 24 samples, using the search formula: ((DBP) OR (MBP) AND (testis)). We included 8 examples in our investigation, whether they had been exposed to DBP or not. Then, 259 driver, suppressor, and marker genes related to ferroptosis were downloaded from FerrDb.
Identification of DEGs related to ferroptosis
GEO2R online tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/) based on the R software “limma” package was used to identify DEGs between the samples expose to DBP or not in peripubertal. Two different groups were defined: Control, testis without exposure to DBP (n = 4); and DBP: testis exposure to DBP (n = 4). The parameter options used were: first, apply adjustment to the P-values: Benjamini & Hochberg (false discovery rate); second, significance level cut-off: P-values < 0.05; and third, DEGs filter criteria: |log fold change| ≥1 and adj. P-value < 0.05. Then DEGs and the ferroptosis-related genes from FerrDb were intersected using ‘VennDiagram’ package in R software. The heatmap of 18 genes were mapped by “heatmap” package which comes from the expression matrix of GEO.
Gene enrichment analysis
Online tools DAVID (https://david.ncifcrf.gov/summary.jsp) was used for enrichment analysis, including cell components (CC), molecular functions (MF), and biological processes (BP), and Kyoto Encyclopedia of Genes and Genomes (KEGG) was used for pathway analysis. Statistically significant criterion was defined as P-value less than 0.05.
Construct PPI network and identify hub genes
Ferroptosis-related DEGs’ PPI network was constructed by online tools STRING (https://cn.string-db.org/). The organism was limited to mouse. The minimum effective binding score was set to 0.4. Next, results were input in Cytoscope (version 3.6.1) to get the top ten hub genes by the “cytoHubba” package.
Experiment validation
Cell line culture and treatment in vitro
TM3 Leydig cell line was purchased from the ATCC (CRL-1714). It was cultured in DMEM (BI, Israel) supplemented with 5% FBS (Gibco, Australia) and 1% penicillin-streptomycin (Gibco, USA). Then, cells were maintained in a humidified incubator at 37°C with 5% CO2.
MBP (MCE, USA), a major bioactive metabolite of DBP in vivo, was used for TM3 Leydig cell treatment in vitro for 24 hr. Ferrostatin-1 (MCE, USA), a ferroptosis inhibitor, was used to inhibit and rescue ferroptosis of TM3 Leydig cells. Ferrostatin-1 was added 16 hr before MBP and cultured with MBP for 24 hr. Angoline (MCE, USA), an inhibitor of IL6/STAT3 signaling pathway, was used to inhibit the expression of IL6/STAT3 signaling pathway in TM3 Leydig cells. Angoline was added 6 hr before MBP and cultured with MBP for 24 hr. All reagents were dissolved in DMSO (Sigma, USA) and controlled below 0.05% in the final concentration.
Cell viability assay
A Cell Counting Kit-8 (Bestbio, Shang Hai, China) was used to acquire a concentration gradient for following experiment. TM3 Leydig was cultured in 96-well plates at a density of 5000 to 10000 cells per well. After treatment, ten microliters of CCK-8 reagent were added to each well. The plates were incubated for two hours in an incubator at 37°C, and optical density (OD) values were measured at 450 nm using a microplate reader. According to the introduction: Cell Viability = (OD treatment-OD blank)/ (OD control-OD blank).
Cellular Fe2+ detection
Cellular Fe2+ detection was performed using an Iron Assay Kit (Abcam, Shang Hai, China). According to the manufacturer’s instructions: Use 96-well plates, standard wells with 100 μL standard dilutions and sample wells with 25 μL samples and added Iron Assay Buffer up to 100 μL. Then, after adding 5 μL Iron Reducer to each standard well and 5 μL of Iron Assay Buffer to each sample well, keep the plate at 37°C for 30 min. Next, add 100 μL Iron Probe to each well and incubate at 37°C for 60 min protected from light. Finally, put the plate in a microplate reader (OD 593 nm).
Cellular MDA detection
The relative concentration of Malondialdehyde in cells was assessed with a Lipid Peroxidation MDA Assay Kit (Beyotime, Shang Hai, China) according to the manufacturer’s instructions: Use standard wells with 100 μL standard dilutions and sample wells with 100 μL protein. Then, add 200 μL MDA detection and mix completely. Next, put them in heat block for 15 min in 100°C. Finally, after the temperature decreases to ambient temperature, centrifuge at 1000 xg for 10 min, take 200 μL of supernatant and add it to a 96-well plate, then measure the absorbance at 532 nm with a microplate reader.
Protein extraction and Western blotting
Cells were lysed in lysis buffer (KG, Jiang Su, China) after washing with PBS for 3 times. The protein concentrations were measured by the BCA assay kit (KG, Jiang Su, China). For Western blotting, lysate proteins were loaded in 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) and were transferred from the gel to polyvinylidene fluoride membranes (Sigma, ISEQ85R, USA). The membranes were washed with TBST after blocking in a solution of 5% skimmed milk for 2 hr (BD, 232110, USA). Then, the membranes were incubated with primary antibodies overnight at 4°C. The following antibodies were used: anti-GPX4 (1:3000, ab125066, Abcam, USA), anti-ACSL4 (1:10000, ab155282, Abcam, USA), and β-actin (1:2000, Proteintech, Wu Han, China, 20536-1-AP). After washing three times for ten minutes, the PVDF membranes were then incubated with HRP-conjugated Affinipure Goat Anti-Rabbit IgG(H+L) (1:4000, Proteintech, Wu Han, China, SA00001-2) at room temperature for one hour. Next, the PVDF membranes were washed again for 3 times every ten minutes. Last, protein bands were visualized using a Super ECL Plus Kit (KG, Jiang Su, China). Then, the photo will deal with Image J and the consequence will divide the gray value of target protein by the gray value of the internal reference protein for normalization.
Isolation of RNA from TM3 cells and quantitative real-time
RNA extraction of cells was performed using TaKaRa MiniBEST Universal RNA Extraction Kit (TaKaRa, Japan). The RNA was transcribed to cDNA using PrimesScript RT Master Mix (TaKaRa, Japan). Quantitative real time polymerase chain reaction (qrt-PCR) was performed using SYBR Premix Ex Taq II (Tli RNaseH Plus) (TaKaRa, Japan) on the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, USA). The relative expression of target genes was calculated with the 2-ΔΔCt algorithm. β-actin, as the reference gene, was used for normalization of target genes. The sequence of primers was: TNF-α (P: GGAC TAGC CAGG AGGG AGAA CAG; R: GCCA GTGA GTGA AAGG GACA GAAC); IL6(P: CTCC CAAC AGAC CTGT CTAT AC; R: CCAT TGCA CAAC TCTT TTCT CA); STAT3(P: CCCC GTAC CTGA AGAC CAAG; R: TCCT CACA TGGG GGAG GTAG); β-actin (P: GGTC ATCA CTAT TGGC AACG; R: ACGG ATGT CAAC GTCA CACT) (Sheng Gong, Shang Hai, China).
Statistical analysis
All data are expressed as the means ± standard deviations (SD). Comparisons between two groups were performed using Student’s t-test. One-way analysis of variance (ANOVA) in combination with LSD test was used for multiple comparisons. P values < 0.05 were considered to indicate a statistically significant difference. SPSS statistics 26.0 software was used for all statistical analyses in this study. In each experiment, at least three replicates were used for statistical analysis.
RESULTS
Ferroptosis-related DEGs
As previously mentioned, we received the GSE13550 chip and processed it using GEO2R to identify DEG (Differentially Expressed Genes) from the GEO database. We acquired 677 DEGs compared to the log2Fold change of control groups (Fig. 1A). In order to identify DEGs associated with ferroptosis, we first eliminated repetitive genes to get 479 unique DEGs, which were then crossed with 259 genes from FerrDb using the “VennDiagram” R package. Then, 18 DEGs with overlap were produced (Fig. 1B). One down-regulated gene, Plin2, and seventeen up-regulated DEGs (Panx1, Tnfaip3, IL6, Atf3, Ripk1, Zeb1, Atf4, Hamp, Stat3, Hmox1, Cdkn1a, Cdo1, Cdo1, II33, Srxn1, Jun, Dusp1, Zfp36) were identified as a consequence (Fig. 1C).
Functional annotation and pathway enrichment analysis
In order to further investigate these associated DEGs, GO and KEGG enrichment analyses were conducted. Consequences of GO BP enrichment (Fig. 2A) demonstrated that ferroptosis-related DEGs were predominantly enriched in negative control of cell proliferation, positive regulation of tumor necrosis factor production, and inflammatory response. This enrichment suggests it may be associated with cell inflammation. For CC results (Fig. 2A), DEGs mainly clustered in RNA polymerase II transcription factor complex, macromolecular complex and transcription factor complex. Hence, these DEGs decode the information in the genome to express a specific and distinct assortment of proteins and RNA molecules in cells. In MF (Fig. 2A), genes are assembled into identical protein binding, transcription regulatory region sequence-specific DNA binding and receptor binding. Therefore, overlapping genes worked to identify proteins and bind between proteins. The top 20 pathways of KEGG enrichment analysis (Fig. 2B) show that it enriched in TNF signaling pathway, inflammatory bowel disease, lipid and atherosclerosis and pathways in cancer. These several enriched pathways are consistent with GO enrichment.

The PPI network is constructed by online public database STRING (Fig. 2C) for the connection of DEGs. The local clustering coefficient is 0.726 and the PPI enrichment P-value is below 1.0e-16. Then, after analyzing of PPI network and hub genes, we obtained the top 10 hub genes and the score of these genes in network. In the outcome (Fig. 2D), when the color of the node is closer to red, the score of the genes is higher. The ranking scores (Table 1) determined that IL6 and STAT3 remained important in DEGs. This suggests that IL6 and STAT3 may exert more control over the ferroptosis induced by MBP in the mouse testis than other genes.
Table 1. The ranking score of TOP 10 DEGs through cytoscope.
| Name |
|
Score |
| IL6 |
interleukin6 |
26 |
| STAT3 |
Signal transducers and activators of transcription 3 |
24 |
| JUN |
Jun Proto-Oncogene |
22 |
| ATF3 |
Activating Transcription Factor 3 |
18 |
| HMOX1 |
Heme Oxygenase 1 |
16 |
| CDKN1A |
Cyclin Dependent Kinase Inhibitor 1A |
12 |
| DUSP1 |
Dual Specificity Phosphatase 1 |
12 |
| TNFAIP3 |
TNF Alpha Induced Protein 3 |
12 |
| ZEB1 |
Zinc Finger E-Box Binding Homeobox 1 |
8 |
| ATF4 |
Activating Transcription Factor 4 |
8 |
Cell viability decreased with MBP
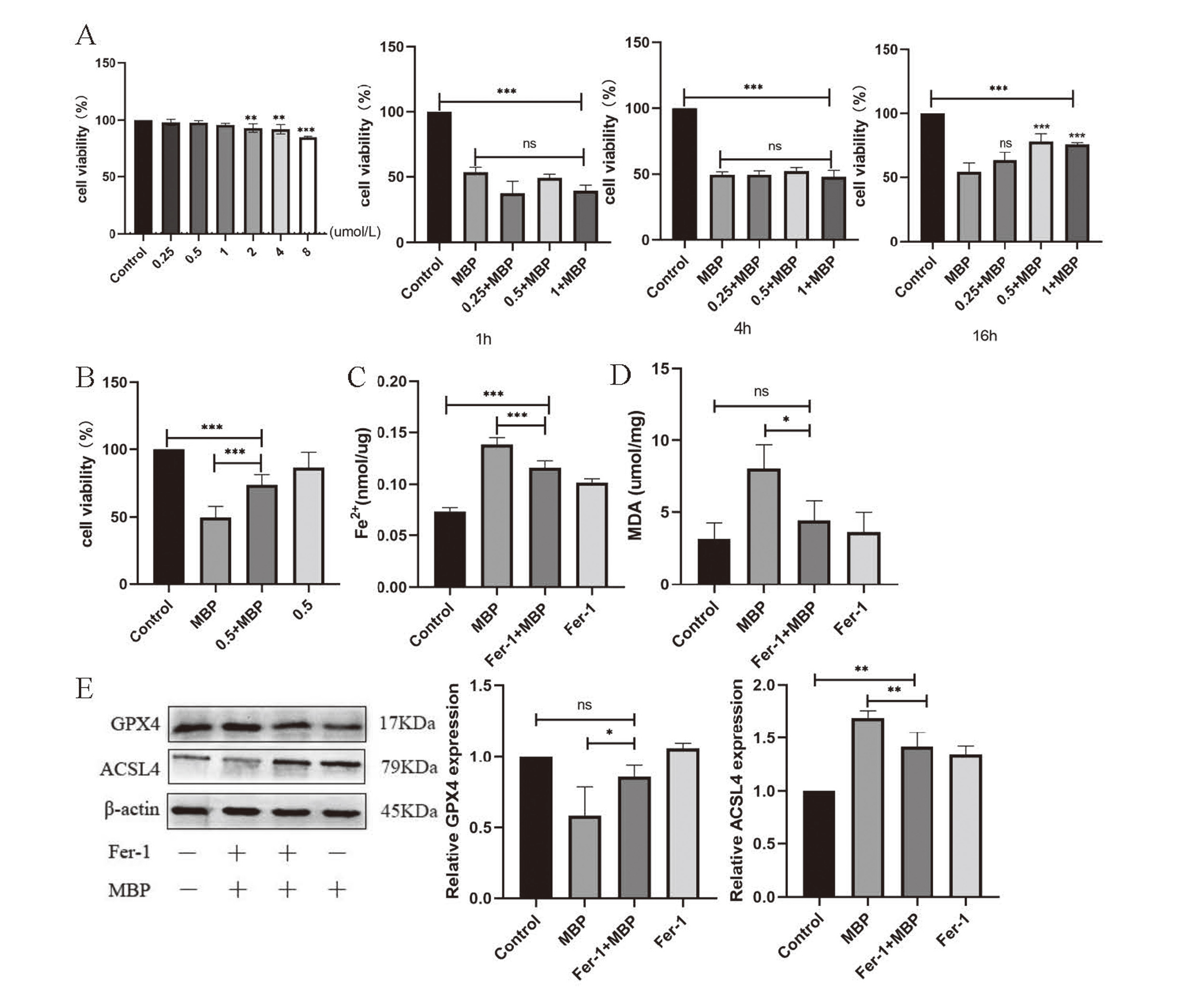
To confirm the outcome of bioinformatics analysis, MBP, a main biometabolite of DBP, was used to explore the molecular mechanism of MBP toxicity. Based on the previous studies, we treated TM3 with proportional concentration (0, 0.625, 1.250, 2.5, 5 and 10 mmol/L). The results (Fig. 3A) indicate that MBP exposure injures viability of TM3 cell. Moreover, as concentration increased, cell viability decreased. Thus, we chose 2.5, 5 and 10 mmol/L as the following concentration based on the IC50 (5173μmol/L) of TM3 with MBP (Fig. 3B).
MBP induced ferroptosis in TM-3 cells
To confirm MBP-induced ferroptosis in TM-3 cells, we evaluated Fe2+ and MDA concentrations (a biomarker of lipid peroxidation). We discovered that an increase in Fe2+ (Fig. 3C) coincided with an increase in MBP concentration in TM3 cells. Meanwhile, the rise in cellular MDA is proportional to the concentration of MBP (Fig. 3D). Using Western blotting, we then detected the expression of ferroptosis-related proteins. The results showed a substantial reduction in the expression of GPX4 (Fig. 3E). However, the dose of MBP increased the upregulation of ACSL4 (Fig. 3E). Results indicate MBP triggered an increase in ACSL4 and a decrease in GPX4 expression, indicating antioxidant system damage and the occurrence of ferroptosis in MBP-induced testis injury.
Ferrostatin-1 inhibition rescued the injury of MBP in TM-3 cells
First, cell viability was measured to explore an appropriate concentration for Ferrostain-1. We tested the viability of TM3 that have been treated with Ferrostain-1 (0.25, 0.5, 1, 2, 4, 8 μmol/L). We found that the concentrations of 2, 4 and 8 μmol/L are harmful to TM3 statistically (Fig. 4A). The reduction in viability at concentrations of 0.25, 0.5, and 1 μmol/L is nearly non-toxic, hence these concentrations were utilized for intervention time screening. Referring to previous studies, 5 mmol/L was selected as a following exposure concentration and 1, 4 and 16 hr were applied to rescue ferroptosis. In contrast with group of MBP, 0.5 μmol/L with 16 hr rescued ferroptosis obviously (Fig. 4B). Next, on the one hand, we evaluated the Fe2+ and MDA in four groups (Fig. 4C and D): control, Ferrostatin-1 (0.5 μmol/L), MBP (5 mmol/L) and MBP+Ferrostatin-1 (5 mmol/L + 0.5 μmol/L). We determined that Fe2+ and MDA were lower in intervening group (MBP+Fer-1) compared with the MBP group. In summary, exposure to MBP led to the accumulation of Fe2+ and MDA, while they were reduced by adding ferroptosis inhibitor. This suggests that ferrostatin-1 minimized the damage caused by MBP. On other hand, we also detected the expression of ferroptosis-related proteins GPX4 and ACSL4. The findings of Western Blot showed that expressions of ACSL4 was down regulated when contrast with MBP group (Fig. 4E). In contrast, GPX4 was increased in regulation. Consequently, the results reveal that Fer-1 mitigates the damage caused by MBP exposure in TM-3 cells, suggesting that MBP promoted ferroptosis in TM-3 cells.
To confirm that the TNF/IL6/STAT3 pathway is involved in MBP-induced testis ferroptosis, an IL6/STAT3 signal pathway inhibitor was administered. According to the previous studies, we tested cell viability of TM-3 cell with Angoline (0.25, 0.5, 1, 2 μmol/L). With 0.25 μmol/L Angoline, CCK-8 showed that it has no obvious impact on the viability of TM-3 cells. Therefore, we chose 0.25 μmol/L for the following interventional concentration. Then, in order to determine how long the intervention should last, we used the intervention at four hours and six hours to select. On the basis of cell viability, we selected Angoline at 0.25 μmol/L for a 6-hr intervention. (Fig. 5A) The concentration of Fe2+ and MDA were all rescued in the Angoline + MBP group (Fig. 5B, C). Next, the expression of ferroptosis-related proteins GPX4 and ACSL4 showed a similar result to that of Angoline rescue of ferroptosis in TM-3 cells (Fig. 5D). Then, we tested the gene expression of signal pathway by qrt-PCR in four groups: control, MBP (2.5 mmol/L, 5 mmol/L and 10 mmol/L). We discovered that MBP increased the expression of TNF-alpha, IL6, and STAT3 (Fig. 5E). In contrast, when Angoline (0.25 μmol/L) was added for 6 hr, TNF-alphaIL6 and STAT3 levels decreased (Fig. 5F). This implies that the inhibitor of the IL6/STAT3 signal pathway mitigated the damage caused by ferroptosis. In conclusion, the TNF/IL6/STAT3 signal pathway is involved in TM3 cell ferroptosis caused by MBP.

DISCUSSION
We discovered a significant signaling pathway and hub genes that contribute to the damage of MBP-induced ferroptosis by analyzing the ferroptosis-related DEGs. Then, our research confirmed that MBP exposure boosts the TNF/IL6/STAT3 signal pathway’s expression, which lowers GPX4 via increasing ACSL4 and leads to lipid peroxidation and ferroptosis in TM-3 cells.
As an endocrine disruptor found in the environment, most DBP is metabolized to MBP in the small intestine by intestinal hydrolases, and then almost all MBP enters the bloodstream (Ozaki et al., 2017). It could permeate testes despite physiological barriers and have an impact on the male reproductive system (de Freitas et al., 2016). With confirmed reproductive toxicity, MBP can continue to do harm to sperm quality and quantity and lead to autophagy, apoptosis and so on (Cui et al., 2021). A study has reported that exposure to DBP can induce inflammation of testicular Sertoli cells by acting NLRP3 inflammasomes (Zhou et al., 2020). In addition, DBP also impairs lipid metabolism and causes inflammation via different kinds of pathways (Xiong et al., 2020). Ferroptosis, a new type of regulated cell death (RCD), was found to be closely related to pathophysiological processes of human disease (Zheng and Conrad, 2020). In recent studies, ferroptosis not only has been found in nervous system diseases, ischemia-reperfusion injury, kidney injury and blood diseases (Li et al., 2020), but also can cause oxidative damage to sperm DNA and testicular oxidative stress (Liu et al., 2022; Bromfield et al., 2019). In 2022, Wu and his colleagues discovered and identified that DEHP exposure, one of the PAEs, causes ferroptosis in mice testes by increasing the amount of HO-1 expression (Wu et al., 2022). Meanwhile, there is robust evidence that DEHP and DBP exposure is linked to outcomes in male reproduction (Radke et al., 2018). Thus, is there a connection between the male reproductive injury due to MBP exposure and ferroptosis? Although some other studies found an association between PAEs and ferroptosis in TM3 cells, it is unclear that the specific mechanism of MBP caused testicular ferroptosis and reproductive disorder.
With the advancement of bioinformatics, the use of bioinformatics analysis in medicine has increased (Azad and Shulaev, 2019). A rising amount of research is using bioinformatics analysis to predict ferroptosis-related genes associated with disease initiation and prognosis. For instance, ferroptosis-related molecular patterns, immunological features in Alzheimer’s disease, prognosis in gastric cancer (He et al., 2022; Wei et al., 2021). Consistent with earlier research, DEGs of ferroptosis genes in TM3 cells were enriched in oxidative stress and inflammation-related biological pathways and activities (Liu et al., 2021b). IL6 and STAT3, as hub genes, are connected with other ferroptosis-related genes closely, which indicates they are critical in the regulation of ferroptosis. In the experiment, as well as Dixon’s research (Dixon et al., 2012), we noticed that MBP caused the downregulation of GPX4, upregulation of Lipid peroxidation and amassing of Fe2+. As a ferroptosis inhibitor, Fer-1 can rescue ferroptosis in many tissues and cells (Liu et al., 2020). Therefore, we pretreated Fer-1 in TM3 cells, and the findings demonstrated that inhibiting ferroptosis prior to MBP injury alleviates the harm produced by MBP. The result of the associated index indicates that MBP induces ferroptosis. Therefore, we infer that ferroptosis occurs in MBP exposure, resulting in testicular damage and perhaps reproductive toxicity.
Then, upon exposure to MBP, bioinformatic analysis indicates the participation of the TNF signal pathway and the significance of the IL6 and STAT3 genes. 30-80% of infertile men have a large concentration of ROS in their ejaculate, according to a study (Agarwal et al., 2019). STAT3 and IL6 induce reactive oxygen species (ROS)-dependent lipid peroxidation and impair iron homeostasis to promote ferroptosis (Wrighting and Andrews, 2006). For another thing, MBP effects the increase of oxidative stress and inflammation (Mondal et al., 2019). However, the exact reason linking the IL6/STAT3 signal route to MBP-induced ferroptosis remains uncertain. To determine if TNF/IL6/STAT3 is involved in MBP-induced ferroptosis, we added proven IL6/STAT3 inhibitor Angoline (Liu et al., 2014). Fe2+, MDA, and ACSL4 protein were significantly increased by MBP, but they were reduced by Angoline. The data show that inhibiting the IL6/STAT3 signaling pathway may relieve ferroptosis. From the above, it is possible to deduce that the inflammatory signal pathway TNF/IL6/STAT3 plays a role in the MBP-induced ferroptosis and male reproductive damage.
In this study, on the one side, we confirmed MBP induced ferroptosis and caused TM-3 cells damage via a key pathway and two hub genes of ferroptosis-related DEGs. Meanwhile, additional signaling pathways and genes are worth investigating. One example is the Plin2 gene, which is the only one to experience downregulation after DBP exposure. Overexpression or knockdown Plin2 can regulate ferroptosis and abnormal lipid metabolism in gastric carcinoma (Sun et al., 2020a). On the other side, we focused on the intracellular rather than mitochondria. Numerous studies have found that the role of mitochondria in ferroptosis is context dependent. It is uncertain if ferroptosis occurs before mitochondrial damage or whether it is a consequence of it, therefore their interaction must be researched further (Otasevic et al., 2021). Mitochondria, the primary site of metabolism, produce large amounts of ROS, play a major role in early stage of ferroptosis process, and amplify ferroptosis signaling under conditions of cysteine starvation or GSH deprivation (Zhao et al., 2022). If the activity of the GPX4 is inhibited, cells undergo ferroptosis independent of mitochondrial function (Gao et al., 2019). Meanwhile, the primary oxidative target sites of DBP assault may be mitochondria (Li et al., 2015). Furthermore, cytosolic GPX4 and mitochondrial GPX4 act as two separate defense systems to quench nonmitochondrial and mitochondrial lipid peroxides respectively (Gan, 2021). Therefore, more research is necessary to determine if MBP induces ferroptosis that depends on mitochondria.
In conclusion, we identified the TNF/IL6/STAT3 signal pathway involved in the regulation of ferroptosis induced by MBP in TM3 cells by bioinformatic analysis and experiment verification. Our study revealed that ferroptosis is closely related to the male reproductive toxicity of MBP. And inflammation-related genes and signal pathway TNF/IL6/STAT3 play an important role in ferroptosis in MBP-exposed TM3 cells, which could help us to the understand the mechanism of MBP and sevre as a potential way to protect male reproduction.
ACKNOWLEDGMENTS
The authors thank all the colleagues in Key Laboratory of Environmental Factors and Chronic Disease Control for their excellent technical assistance and helpful advice. This work was supported by Ningxia Natural Science Foundation(2021AAC03163) and Ningxia Medical University scientific research project.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- Agarwal, A., Baskaran, S., Parekh, N., Cho, C.-L., Henkel, R., Vij, S., Arafa, M., Panner Selvam, M.K. and Shah, R. (2021): Male infertility. Lancet, 397, 319-333.
- Agarwal, A., Parekh, N., Panner Selvam, M.K., Henkel, R., Shah, R., et al. (2019): Male Oxidative Stress Infertility (MOSI): Proposed Terminology and Clinical Practice Guidelines for Management of Idiopathic Male Infertility. World J. Mens Health, 37, 296-312.
- Amir, S., Shah, S.T., Mamoulakis, C., Docea, A.O., Kalantzi, O.I., Zachariou, A., Calina, D., Carvalho, F., Sofikitis, N., Makrigiannakis, A. and Tsatsakis, A. (2021): Endocrine Disruptors Acting on Estrogen and Androgen Pathways Cause Reproductive Disorders through Multiple Mechanisms: A Review. Int. J. Environ. Res. Public Health, 18.
- Azad, R.K. and Shulaev, V. (2019): Metabolomics technology and bioinformatics for precision medicine. Brief. Bioinform., 20, 1957-1971.
- Bromfield, E.G., Walters, J.L., Cafe, S.L., Bernstein, I.R., Stanger, S.J., Anderson, A.L., Aitken, R.J., McLaughlin, E.A., Dun, M.D., Gadella, B.M. and Nixon, B. (2019): Differential cell death decisions in the testis: evidence for an exclusive window of ferroptosis in round spermatids. Mol. Hum. Reprod., 25, 241-256.
- Brenner, D., Blaser, H. and Mak, T.W. (2015): Regulation of tumour necrosis factor signalling: live or let die. Nat. Rev. Immunol., 15, 362-374.
- Cui, Y., Zhang, X., Yin, K., Qi, X., Zhang, Y., Zhang, J., Li, S. and Lin, H. (2021): Dibutyl phthalate-induced oxidative stress, inflammation and apoptosis in grass carp hepatocytes and the therapeutic use of taxifolin. Sci. Total Environ., 764, 142880.
- Dixon, S.J., Lemberg, K.M., Lamprecht, M.R., Skouta, R., Zaitsev, E.M., Gleason, C.E., Patel, D.N., Bauer, A.J., Cantley, A.M., Yang, W.S., Morrison, B. 3rd and Stockwell, B.R. (2012): Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell, 149, 1060-1072.
- de Freitas, A.T., Ribeiro, M.A., Pinho, C.F., Peixoto, A.R., Domeniconi, R.F. and Scarano, W.R. (2016): Regulatory and junctional proteins of the blood-testis barrier in human Sertoli cells are modified by monobutyl phthalate (MBP) and bisphenol A (BPA) exposure. Toxicol. In Vitro, 34, 1-7.
- Gan, B. (2021): Mitochondrial regulation of ferroptosis. J. Cell Biol., 220.
- Gao, C.J., Liu, L.Y., Ma, W.L., Ren, N.Q., Guo, Y., Zhu, N.Z., Jiang, L., Li, Y.F. and Kannan, K. (2016): Phthalate metabolites in urine of Chinese young adults: Concentration, profile, exposure and cumulative risk assessment. Sci. Total Environ., 543 (Pt A), 19-27.
- Gao, M., Yi, J., Zhu, J., Minikes, A.M., Monian, P., Thompson, C.B. and Jiang, X. (2019): Role of Mitochondria in Ferroptosis. Mol. Cell, 73, 354-363.e3.
- He, Y.J., Cong, L., Liang, S.L., Ma, X., Tian, J.N., Li, H. and Wu, Y. (2022): Discovery and validation of Ferroptosis-related molecular patterns and immune characteristics in Alzheimer’s disease. Front. Aging Neurosci., 14, 1056312.
- Jin, H., Ma, T., Sha, X., Liu, Z., Zhou, Y., Meng, X., Chen, Y., Han, X. and Ding, J. (2021): Polystyrene microplastics induced male reproductive toxicity in mice. J. Hazard. Mater., 401, 123430.
- Li, F.M., Wu, M., Yao, Y., Zheng, X., Zhao, J., Wang, Z.Y. and Xing, B.S. (2015): Inhibitory effects and oxidative target site of dibutyl phthalate on Karenia brevis. Chemosphere, 132, 32-39.
- Li, H. and Spade, D.J. (2021): Reproductive Toxicology: Environmental exposures, fetal testis development and function: phthalates and beyond. Reproduction, 162, F147-F167.
- Li, J., Cao, F., Yin, H.-L., Huang, Z.-J., Lin, Z.-T., Mao, N., Sun, B. and Wang, G. (2020): Ferroptosis: past, present and future. Cell Death Dis., 11, 88.
- Liu, J., Zhang, Q., Ye, Y., Li, W., Qiu, J., Liu, J., Zhan, R., Chen, W. and Yu, Q. (2014): Angoline: a selective IL-6/STAT3 signaling pathway inhibitor isolated from Zanthoxylum nitidum. Phytomedicine, 21, 1088-1091.
- Liu, P., Feng, Y., Li, H., Chen, X., Wang, G., Xu, S., Li, Y. and Zhao, L. (2020): Ferrostatin-1 alleviates lipopolysaccharide-induced acute lung injury via inhibiting ferroptosis. Cell. Mol. Biol. Lett., 25, 10.
- Liu, Q., Ma, J.Y. and Wu, G. (2021a): Identification and validation of a ferroptosis-related gene signature predictive of prognosis in breast cancer. Aging (Albany NY), 13, 21385-21399.
- Liu, T., Li, X., Cui, Y., Meng, P., Zeng, G., Wang, Y. and Wang, Q. (2021b): Bioinformatics Analysis Identifies Potential Ferroptosis Key Genes in the Pathogenesis of Intracerebral Hemorrhage. Front. Neurosci., 15, 661663.
- Liu, Y., Cao, X., He, C., Guo, X., Cai, H., Aierken, A., Hua, J. and Peng, S. (2022): Effects of Ferroptosis on Male Reproduction. Int. J. Mol. Sci., 23.
- Ma, Y., He, X., Qi, K., Wang, T., Qi, Y., Cui, L., Wang, F. and Song, M. (2019): Effects of environmental contaminants on fertility and reproductive health. J. Environ. Sci. (China), 77, 210-217.
- Meng, P., Zhang, S., Jiang, X., Cheng, S., Zhang, J., Cao, X., Qin, X., Zou, Z. and Chen, C. (2020): Arsenite induces testicular oxidative stress in vivo and in vitro leading to ferroptosis. Ecotoxicol. Environ. Saf., 194, 110360.
- Mondal, S., Ghosh, S., Bhattacharya, S. and Mukherjee, S. (2019): Chronic dietary administration of lower levels of diethyl phthalate induces murine testicular germ cell inflammation and sperm pathologies: involvement of oxidative stress. Chemosphere, 229, 443-451.
- Ng, S.-W., Norwitz, S.G. and Norwitz, E.R. (2019): The Impact of Iron Overload and Ferroptosis on Reproductive Disorders in Humans: implications for Preeclampsia. Int. J. Mol. Sci., 20.
- Otasevic, V., Vucetic, M., Grigorov, I., Martinovic, V. and Stancic, A. (2021): Ferroptosis in Different Pathological Contexts Seen through the Eyes of Mitochondria. Oxid. Med. Cell. Longev., 2021, 5537330.
- Ozaki, H., Sugihara, K., Watanabe, Y., Moriguchi, K., Uramaru, N., Sone, T., Ohta, S. and Kitamura, S. (2017): Comparative study of hydrolytic metabolism of dimethyl phthalate, dibutyl phthalate and di(2-ethylhexyl) phthalate by microsomes of various rat tissues. Food Chem. Toxicol., 100, 217-224.
- Qiu, Y., Cao, Y., Cao, W., Jia, Y. and Lu, N. (2020): The Application of Ferroptosis in Diseases. Pharmacol. Res., 159, 104919.
- Radke, E.G., Braun, J.M., Meeker, J.D. and Cooper, G.S. (2018): Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int., 121, 764-793.
- Sedha, S., Lee, H., Singh, S., Kumar, S., Jain, S., Ahmad, A., Bin Jardan, Y.A., Sonwal, S., Shukla, S., Simal-Gandara, J., Xiao, J., Huh, Y.S., Han, Y.-K. and Bajpai, V.K. (2021): Reproductive toxic potential of phthalate compounds - State of art review. Pharmacol. Res., 167, 105536.
- Shaygannia, E., Nasr-Esfahani, M.H., Sotoodehnejadnematalahi, F. and Parivar, K. (2021): Is ferroptosis involved in ROS-induced testicular lesions in a varicocele rat model? Basic Clin. Androl., 31, 10.
- Sun, X., Yang, S., Feng, X., Zheng, Y., Zhou, J., Wang, H., Zhang, Y., Sun, H. and He, C. (2020a): The modification of ferroptosis and abnormal lipometabolism through overexpression and knockdown of potential prognostic biomarker perilipin2 in gastric carcinoma. Gastric Cancer, 23, 241-259.
- Sun, Y., Chen, P., Zhai, B., Zhang, M., Xiang, Y., Fang, J., Xu, S., Gao, Y., Chen, X., Sui, X. and Li, G. (2020b): The emerging role of ferroptosis in inflammation. Biomed. Pharmacother., 127, 110108.
- Vogt, A.S., Arsiwala, T., Mohsen, M., Vogel, M., Manolova, V. and Bachmann, M.F. (2021): On Iron Metabolism and Its Regulation. Int. J. Mol. Sci., 22.
- Wang, B., Qin, X., Xiao, N., Yao, Y., Duan, Y., Cui, X., Zhang, S., Luo, H. and Sun, H. (2020): Phthalate exposure and semen quality in infertile male population from Tianjin, China: associations and potential mediation by reproductive hormones. Sci. Total Environ., 744, 140673.
- Wei, J., Zeng, Y., Gao, X. and Liu, T. (2021): A novel ferroptosis-related lncRNA signature for prognosis prediction in gastric cancer. BMC Cancer, 21, 1221.
- Wrighting, D.M. and Andrews, N.C. (2006): Interleukin-6 induces hepcidin expression through STAT3. Blood, 108, 3204-3209.
- Wu, Y., Wang, J., Zhao, T., Chen, J., Kang, L., Wei, Y., Han, L., Shen, L., Long, C., Wu, S. and Wei, G. (2022): Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J. Hazard. Mater., 426, 127807.
- Xie, F., Chen, X., Weng, S., Xia, T., Sun, X., Luo, T. and Li, P. (2019): Effects of two environmental endocrine disruptors di-n-butyl phthalate (DBP) and mono-n-butyl phthalate (MBP) on human sperm functions in vitro. Reprod. Toxicol., 83, 1-7.
- Xiong, Z., Zeng, Y., Zhou, J., Shu, R., Xie, X. and Fu, Z. (2020): Exposure to dibutyl phthalate impairs lipid metabolism and causes inflammation via disturbing microbiota-related gut-liver axis. Acta Biochim. Biophys. Sin. (Shanghai), 52, 1382-1393.
- Yilmaz, B., Terekeci, H., Sandal, S. and Kelestimur, F. (2020): Endocrine disrupting chemicals: exposure, effects on human health, mechanism of action, models for testing and strategies for prevention. Rev. Endocr. Metab. Disord., 21, 127-147.
- Zhang, Y.J., Guo, J.L., Xue, J.C., Bai, C.L. and Guo, Y. (2021): Phthalate metabolites: Characterization, toxicities, global distribution, and exposure assessment. Environ. Pollut., 291, 118106.
- Zhao, L., Zhou, X., Xie, F., Zhang, L., Yan, H., Huang, J., Zhang, C., Zhou, F., Chen, J. and Zhang, L. (2022): Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. (Lond.), 42, 88-116.
- Zheng, J. and Conrad, M. (2020): The Metabolic Underpinnings of Ferroptosis. Cell Metab., 32, 920-937.
- Zhou, Y., Ma, T., Yan, M., Meng, X., Wu, J., Ding, J., Han, X. and Li, D. (2020): Exposure of DBP in gestation induces inflammation of testicular Sertoli cells in progeny by activating NLRP3 inflammasomes. Sci. Total Environ., 707, 136139.





